Unique Identification: The Key to Enhancing the Traceability of Medical Instruments
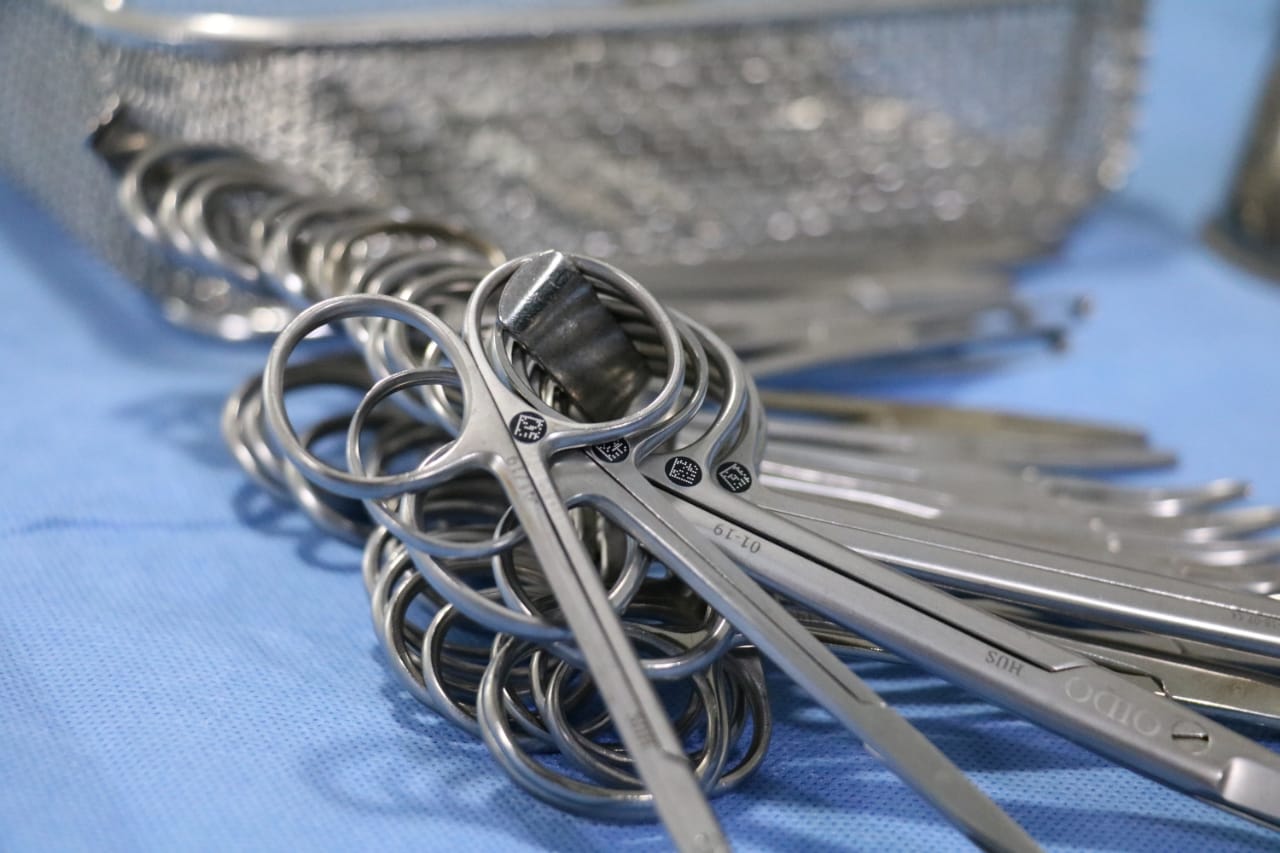
The implementation of unique identification systems is redefining control and safety in surgical instruments. Integrated with Interlab’s software, these systems enable precise digital management from washing to the patient.
In the world of surgical traceability, precision and reliability allow no margin for error. Every instrument, no matter how small, plays a decisive role in the operating room. That’s why the individual identification of each instrument has become an essential step toward achieving complete control over medical devices and optimizing sterilization center processes.
Unlike traditional methods — such as color-coding systems — modern unique identification solutions allow each instrument to be assigned a specific code, whether through laser engraving, micro-labels, or radiofrequency. These codes are applied in a way that does not affect the structure or sterilization capacity of the instruments.
This “digital fingerprint” enables instant identification at any stage of the workflow. From washing and visual inspection to sterilization, storage, and operating room use, each piece can be tracked accurately and without confusion.
Smart Traceability: When Identification Meets Software
The true value of unique identification emerges when it is integrated into a digital management system. Interlab’s software for surgical instrument and medical device traceability and management allows each code to be linked to a complete record of the instrument — including its origin, use, washing cycle, sterilization date, process operator, and associated patient.
Each scan automatically updates the system’s database, creating complete, verifiable, real-time traceability. This gives hospitals and clinics a comprehensive view of their instruments, allowing them to analyze performance, usage frequency, and operational status.
More Safety, Efficiency, and Control
Unique identification not only reinforces patient safety but also enhances the operational efficiency of sterilization centers. It enables quick detection of losses or damage, supports preventive maintenance, and optimizes the configuration of surgical sets based on real usage data.
Moreover, complete traceability of each instrument facilitates audits and compliance with international standards that require documented, controlled, and verifiable processes. The combination of unique identification and Interlab’s software ensures these goals are achieved with precision and reliability.
Innovation Transforming Hospital Practice
In a healthcare environment where digitalization is redefining clinical management, unique identification acts as a bridge between the physical handling of instruments and their digital control. Its implementation is a strategic investment: it improves traceability, reduces risks, extends the lifespan of materials, and strengthens decision-making through concrete data.
Each uniquely identified instrument represents a step forward toward intelligent sterilization centers and safer, more efficient, and more sustainable hospitals.
When this innovation is combined with the analytical and operational power of Interlab’s software, traceability reaches a new level — setting a benchmark of excellence in surgical care.



